Our ISO 9001:2015 Quality Manual Templates contain everything you need to create your own quality manual and get the certification you desire. However, to take it one step further, we also offer clause-by-clause interpretation (see sample) and guidance on Documented Information, Internal Audits, Management Reviews and Quality Policy & Objectives; so you can see exactly what you need to do to achieve compliance.
These 88 pages take all the guesswork out of completing your ISO 9001 so that you can get down to business as efficiently and effectively as possible.
Product Comparison Matrix
First Time
Implementation | Transition | Refresh |
$299
| $349
|
| Who is this for? | Companies who wish to gain ISO 9001 certification for the first time.
| Companies already ISO 9001:2008 certified who wish to make the transition to ISO 9001:2015. | Companies who wish to refresh their documentation - perhaps for an update or surveillance audit. |
Quality Manual Template
with quality policy and objectives
- view sample Quality Manual Template |
$75
|
10 Documented Procedures
- view sample procedure - Control of Documented Information
- Control of Risks & Opportunities
- Control of Competence & Awareness
- Control of Calibrated Equipment
- Control of Design & Development
- Control of Purchasing & Procurement
- Control of Non-conformity & Corrective Action
- Control of Customer Satisfaction
- Control of Internal Audits
- Control of Management Reviews
|
$175
|
19 Process Maps, Turtle Diagrams, Matrix
- view sample process map - Management Processes
- Customer Related Processes
- Support Processes
- Assessment Process
- Control of Documented Information Process Activity Map
- Control of Risks & Opportunities Process Activity Map
- Control of Competence & Awareness Process Activity Map
- Control of Calibrated Equipment Process Activity Map
- Calibrated Equipment Process Map
- Control of Design & Development Process Activity Map
- Control of Purchasing & Procurement Process Activity Map
- Control of Non-conformity & Corrective Action Process Activity Map
- Non-conformity & Corrective Action Process Map
- Control of Customer Satisfaction Process Activity Map
- Customer Complaints Process Map
- Control of Internal Audits Process Activity Map
- Internal Audit Process Map
- Control of Management Reviews Process Activity Map
- Management Review Process Map
| (FREE with 10 Procedures) |
27 Reports, Forms & Logs
- view sample form - Master Document & Record Index
- Document Issue Sheet
- Document Change Request
- Risk & Opportunity Register
- SWOT Analysis Template
- PESTLE Analysis Template
- Competency Review
- Training Attendance
- Training Evaluation
- Controlled Equipment Log
- Calibration Log
- Software Validation Log
- Requirements Review Checklist
- Design Change Request
- Design Change Request Log
- Approved Supplier Index
- Supplier Evaluation
- Receiving Inspection Log
- Non-conformance Report
- Concession Request
- Concession Request Log
- Corrective Action Report
- Customer Satisfaction Survey
- Customer Feedback Log
- Internal Audit Report
- Internal Audit Feedback
- Management Review Agenda & Minutes
| (FREE with 10 Procedures) |
3 Audit Checklists
- view sample checklist - Internal Audit Checklist
- Process Audit Checklist
- Supplier Audit Checklist
|
Internal Audit, Non-Conforming & Corrective Action Tracker
- view sample tracker
|
Gap Analysis Checklist
- view sample Gap Analysis |
Implementation Checklist
- view sample Implementation Checklist |
Transition Plan |
Transitioning Planning Guidance
- view sample |
| ISO 9001:2015 v 2008 Correlation Matrix |
5 Guidance Documents (88 pages) - Internal Audit Guidance
- Documented Information Guidance
- Management Review Guidance
- Quality Policy, Objectives & Indicators Guidance
- Clause-by-clause Interpretation
- view sample interpretation
|
12-months free support 12-months free updates Unlimited users within company |
We accept most payment methods including
and Wire transfer / BACS | Quality Manual Template First Time Implementation
$299
| Quality Manual Template Transition from
ISO 9001:2008 $349
|
- Immediate download
- Written in International English
- Fully-editable MS Word or Excel files
- All the templates use styles – making reformatting and rebranding a breeze
PDF Samples from the Quality Manual Template
ISO 9001 Quality Manual ~ Everything You Need to Know
What is a Quality Manual?
The quality manual documents an organization’s quality management system (QMS). The Quality Manual demonstrates the commitment to meeting customer expectations delivering quality products or services.
The quality manual is usually the first document created during the process of establishing a Quality Management System (QMS) for the ISO standard. It can then be used as a guidebook for your organization to follow when implementing the QMS.
More specifically, a quality manual “states the company’s intentions for operating the processes within the Quality Management System.” The manual includes information about the organization’s goals, expectations, policies, and more. The manual also includes requirements needed for the organization to be compliant with the ISO 9001 standard.
Is A Quality Manual Required For ISO 9001?
Many organizations are faced with the question of whether or not it’s worth trying to develop such a seemingly complicated document for their business. This is because a quality manual is not usually a requirement of most Quality Management Systems.
ISO 9001 standards also states that a Quality Manual is not required — it is not a mandatory document.
Learn more about the ISO 9001 requirements and mandatory documents.
However, many businesses opt to write one as it really is useful and has many benefits; it keeps management accountable, communicates to employees and business partners, as well as keeping the QMS in-line with ISO 9001 standards.
Benefits Of A Manual
The quality manual provides a wide range of benefits for organizations. Not only is it organized and easy to follow, but it also covers almost everything you and your employees will need to know about the ISO 9001 standard and what is expected of your quality management system.
Some of the other benefits the manual offers organizations includes:
- Improved quality management systems
- Easier transition to ISO 9001 operations
- Easier adjustment to updated ISO 9001 standards
- Guidance in increasing efficiency and improving processes
How Is The Quality Manual Useful?
The manual is primarily used as a source of information regarding your quality management system and ISO certification. However, it also provides the following information:
- Sets expectations for both management and employees
- Steps on implementing the new QMS
- Guidance on having clear and concise communication throughout the organization’s documents and between departments
- Provides company context for internal, customer, and ISO certification body auditors new to the organization
- Demonstrates the organization’s plan to conform to the latest ISO 9001 standard
- Demonstrates ISO 9001’s Clause 5.3 in that all organizational roles, responsibilities, and authoritieshave been assigned, communicated, and understood
- Serves as a form of evidence for auditors — those doing certification audits, the lead auditor and internal auditor(s)
Does It Matter How Big The Quality Manual Is?
No.
The size and complexity of your organization and its individual processes will determine the volume of information required to successfully convey how the quality system works and what controls are in place to manage each process.
We've prepared 10-page quality manuals and others with more than 150 pages. Each manual was certified because it met the requirements not because they were a certain page length.
My Boss Wants a 4 Page Quality Manual — What Should I Do?
Manage expectations — most organizations prepare a quality manual that covers the requirements of the international standard, includes or makes reference to the necessary operational procedures and outlines the structure of the quality system. It would be difficult address all the requirements in 3 or 4 pages.Most quality manuals are typically 30 or 40 pages in length. Our quality manual template addresses these requirements in 20 pages of text, while the procedures (seperate documents) carry the burden of defining how compliance is achieved at an operational level.
PRO TIP! Ask your business partners, suppliers and customers for a PDF copy of their quality manual if they are ISO 9001 certified. It might be useful to go through these with your colleagues (Boss / Top Management) — particularly those new to ISO 9001. It will help manage their expectations.
Length
Length is always tricky for a quality manual. You don’t want to overwhelm readers with a ton of information, but you also want to ensure that all of the most important areas of your manual are covered, so there is no confusion left by the end.
It is recommended to try to keep your quality manual on the shorter side, as it will do a better job of keeping attention spans longer. Also, you may add certain features to help provide understanding or information about your organization, such as a mission statement.
Quality Manual Template
Although ISO 9001 quality manuals are unique to each organization, they all follow the same ISO requirements — thus a template is ideal. Some of the more common ISO templates include:
- Quality Manual
- Policy statement
- Objectives
- Introduction & Scope
- Quality Management Principles
- References and Definitions
- Context of the Organization
- Leadership
- Management System Planning
- Support
- Operation
- Performance Evaluation
- Improvement
- Appendices
- Documented Procedures
- Preventive Actions
- Corrective Actions
- Control of Non-conforming Products
- Control of Documents
- Control of Records
- Internal Audit
- Other Operational Procedures
- Process Maps
- Reports
- Forms
- SWAT and PESTLE analysis
Many online Quality Manual Templates include other useful tools for ISO certification, such as Internal Audit tools, a Gap Analysis tool, Project Plan etc.
Benefits Of Using A Quality Manual Template
Quality management system templates are widely available for any and every business that may want a helping hand in getting their system up and running. You may feel that a template is not for you and will not fit your organization — but your really should consider them, they bring plenty of benefits.
Faster Start
In the beginning, there is a lot of time and attention required in learning all that goes into a quality management system. Generally, it takes more than one person to research and figure out what is required of your business and how to get everything started. Using a template gives you a fantastic starting point, allowing you to jump right into the process rather than spending time worrying about how to go about it.
Pick and Choose
When it comes to implementing a ISO 9001, there are requirements which apply to your business and some which do not. Having a template that already spells out the requirements for you will make it that much easier to take away the ones that don't apply.
Save Time
Documenting your quality management system can be a long process that requires a lot of time from any business. Small businesses don't always have a lot of extra time as they have fewer employees to dedicate solely to the implementation process. Using templates can jump-start the process and save you a lot of time in putting everything together on your own.
Cost-Effective
Using templates to set up a quality management system can save you time but also money. Using fewer hours at the start is a great way to cut costs.
On top of that, you won't have to hire an outside consultant to make sure you are doing everything correctly. Figuring out your quality management system in-house is a cheaper option, and a template makes that process faster and easier to get through.
Lower Work Load
Small businesses may not have the same kind of manpower that a large scale company boasts. Every employee is crucial to the success of the business and has their own part of everything to focus on. When you use a quality management system template, it takes away some of the load that might be placed on your employees. By already having a framework in place, there is less work for everyone to do.
Steady Foundation
In the beginning, implementing a quality management system can feel like too big of a change to wrap your mind around. This is a big concern for small businesses that don't want to rock the boat too hard and risk it capsizing.
Using a quality manual template gives you a solid foundation to start building that change on. You don't have to worry so much that everything will topple over when you know what you are building on is already proven to work.
Consistency
For any business, consistency is key. A quality manual template helps to prevent anything from getting out of line by giving you a foundation for everything within your system.
Having this foundation means that no matter who is involved in the quality management system will follow the same approach. You don't have to worry about everyone doing things their own way when they have a guide to follow.
What Should Be Included In A Quality Manual?
The ISO 9001 standard and requirements are very clear. It is therefore easier for all involved (internal and external — such as business partners, suppliers, as well as ISO auditors) if the quality manual’s table of contents is similar to or mirrors the requirements. As such, we recommend:
Quality Manual Contents
- Introduction & Scope
- Quality Management Principles
- References and Definitions
- Context of the Organization
- Leadership
- Management System Planning
- Support
- Operation
- Performance Evaluation
- Improvement
- Appendices
What About Different Industries or Businesses?
The quality manual contents will be the same, no matter what industry you're in. If you're a small one-man business or a large manufacturing or pharmaceutical, ISO 9001's requirements are the same; the difference will be in your operating procedures.
Looking for a Quality Manual Template?
Our Quality Manual Template is proven to work.
Introduction, Scope & Quality Management Principles
The introduction of the quality manual introduces you to both the ISO 9001 standard and the Scope of the manual itself. The quality management principles section will cover the core principles that drive ISO 9001 operations, in addition to your quality management system.
Scope of the Quality Management System
You should include the boundaries of the new quality management system in your quality manual. Mention the types of products or services your organization offers, in addition to the industry you work in.
Also, make sure you mention exclusions for your business, rather than omitting them entirely. Some ISO 9001:2015 requirements, for example, may not apply to your specific organization, such as Clause 7.3 Design and Development.
References And Definitions
This section of the quality manual offers a glossary of terms that will be mentioned throughout the remainder of the manual.
Context Of The Organization
The context of the organization section has a lot of information discussing various types of issues that may arise while implementing or updating the quality management system. This section also features strategies that can help overcome such issues.
Internal Issues
Internal issues are problems found within the organization itself. Some internal problems can be related to the following:
- Products
- Services
- Planning
- Leadership
- Workforce size
A SWOT analysis (Strengths, Weaknesses, Opportunities, and Threat Analysis) can be performed by an organization in order to get a baseline of where they stand in comparison to where they want to be after implementing the new QMS.
The results from a SWOT analysis also helps provide insight into what an organization’s current internal values are, which can include:
- Performance
- Knowledge
- Culture
- Competence
- Strategy
- Values
External Issues
External issues are problems that exist outside of your organization and can be much more challenging to overcome. External problems are usually associated with requirements that need to be met within your industry, in technology, globalization, and more.
Similar to how SWOT is used for internal issues, a PESTLE analysis (Political, Economic, Social, Technological, Legal and Environmental Analysis), is used to assess external forces that can affect your organization. The PESTLE analysis can also help businesses understand their target market and improve growth.
Your adopted quality management system should use both the SWAT and PESTLE analysis to continue monitoring potential internal or external conflicts.
Leadership
Leadership is one of the biggest principles expressed by the ISO 9001 standard. This is who is responsible for making sure that the development and implementation of the policies regarding your quality management system are going according to plan.
Top Management are responsble for overseeing and implementing your organization’s new QMS and control of documents. Some of their responsibilities will include directing strategies and providing communication in processes and performance.
They will also need to make sure that resources for ISO implementation are allocated to areas that need more focus on improvement and responsibilities are distributed to the right people or departments.
For example, leadership may need to ensure that the quality manager has all of the necessary resources — such as finances or technology — to initiate processes and complete them successfully.
Leadership in the PDCA (Plan-Do-Check-Act) Cycle
In the PDCA (plan-do-check-act) cycle, there are four different phases necessary for leaders to go through. These steps are:
- Plan - This is when strategies are developed to implement or improve the quality management system. Leaders must make sure that these strategies are followed through.
- Do - Leaders must ensure that each step of the developed strategy is performed correctly. It is in this phase where they must check resources and the distribution of tasks to ensure it aligns with QMS needs. They may also monitor improvement, assess risks or problems, or review the outcome of recent QMS changes.
- Check - This is essentially a review of everything that was done during the “Do” step. This will include checking the system’s performance, making sure that it is working properly, and changes that have been put in effect. Policies and other documentation will also be under review.
- Act - The final part of the cycle is acting. This phase involves making sure that all changes made for improvement have been put into place, as well as removing any processes or changes that previously provided negative results.
Governance
The governance structure of an organization is also a large part of the quality manual’s leadership chapter. This section of the manual should discuss what the company defines as “proper support” in order to make sure processes are able to complete objectives under the ISO 9001 standards of quality.
The governing leaders of the organization will also be responsible for verifying the system itself, usually through the form of internal audits and reviewing data.
Leaders with this responsibility should look at current trends as well as looking out for risks that the organization should plan to overcome. Most importantly they should continuously monitor the effectiveness of the QMS system in addition to any improvements or success it achieves.
Management System Planning
This next chapter in the quality manual is focused on management system planning. In order to have success in this area, there are a number of features to assess, including internal and external connections, risks/issues, successes, and opportunities that may arise.
The best QMS will consider maintaining a balance between the number of risks versus opportunities available from certain processes. This balance must be managed internally in order to achieve effectiveness.
Risks and opportunities will need to be reviewed in the following contexts:
- Internal context
- Stakeholders
- External context
- QEHS management system
Risk Evaluation Cycle
The risk evaluation cycle is one way for leaders to assess risks regularly. The cycle includes the following steps:
- Plan
- Identify
- Assess
- Respond
- Review
- Report
- Monitor
- Repeat
Following this cycle will help your organization actively recognize problems before they become problems and create proactive strategies to combat them.
Top Management
Top Management is responsible for ensuring that a risk-based style of thinking is implemented within your organization. They are in charge of establishing procedures and planning around risk management and make sure that your organization’s principles are included in your quality management system.
Top Management will also be expected to provide the resources necessary to cover any processes within their departments, as well as planning and assigning responsibilities for ISO certification.
Support
The next chapter that is covered in the quality manual is support. Support can come in many forms, including customer support, financial support, and even human resources. The goal of support is to ensure improvements are made in some of the following areas:
- Customer satisfaction
- Employee satisfaction
- Human resources
- Financial resources
- Working area
The biggest area of focus in this section is customer satisfaction, which is critical to any company’s success. Without customer satisfaction, your business risks profit losses in addition to a reduced customer base and market. On the positive side, having good customer satisfaction leads to higher spending, more frequent customers; in addition to an increased chance of bringing in new customers. This will eventually result in higher profits.
General
General support includes resources such as human resources and financial resources, as well as the working environment. Making sure your employees are satisfied with their work environment is important. With comfort, efficiency, and productivity in the workplace, your employees will produce more quality products and services. Your customers will thank you later!
People
Job descriptions are designed to include all of the qualifications and experience your organization requires from ideal candidates for a specific position. Descriptions also include a general list of responsibilities that come with the role. Creating an accurate job description will help you filter out candidates much more effectively, saving time and effort both for applicants and hiring managers.
One reason why human resources is required in the hiring process is that they often hold records for employee qualifications. They also have documentation surrounding training materials new employees will need in order to perform their roles effectively and efficiently. Having human resources involved makes the hiring process go more smoothly.
Infrastructure
Quality Manual For Manufacturing
Organizations need building blocks and blueprints to be successful, and that is exactly what the infrastructure section of the quality manual does a great job of covering.
Providing necessary resources to departments and maintaining them are important for a successful working environment, whether it be physical workspaces or software. Quality managers and facility managers will often be the brains behind this process, responsible for making sure operations are completed successfully.
Operation
Managing operations is one of the hardest parts of implementing a new quality management system, as it requires a lot of time and energy to ensure its success. While your organization plans operations, certain operation details will need to be reviewed, including:
- Objectives and requirements for the product or service
- Verification, validation, monitoring, inspection, and test requirements
- Documented information to demonstrate conformity
- Related life risks and opportunities
- Documented information to demonstrate conformity and control of nonconforming products
- Necessary resources or outsourced processes and their controls
- Criteria for process performance and product/service acceptance
- Potential consequences and mitigation to change affecting input requirements
- Resources necessary to support the ongoing operation and maintenance of the product
Customer Requirements
Meeting customer requirements is crucial. As mentioned earlier, making sure that customers are satisfied with what they receive from your business — whether it be a product or service — is important to a company’s success.
Customer complaints should be documented and fed into the continuous improvement process or control of nonconforming process.
In fact, the ISO recommends that your customers’ expectations should be exceeded to ensure continual growth and success.
Communication
Keeping communication active between your organization and customers will help improve satisfaction and help reduce any concerns they may have. The customer service team and the sales and marketing department are both responsible for ensuring a line of communication always exists between you and the customer. There are many ways you can maintain communication with customers, including:
- Email
- Letters, direct mail, response forms
- Surveys
- Website feedback
- Customer services
- Social Media
- Apps
Of course, with emerging new technology and social media, it is important for organizations to keep up-to-date with how your customers would like to communicate with you. Make it easy for them using the channel they best prefer.
Performance Evaluation
Owners are always anxious to see performance evaluations. However, it is important to review your business’s performance rate routinely, so you know which facets of your quality management system are working correctly — and which aren’t, so you can make improvements later.
There are various methods of developing and responding to performance evaluation, including looking trends that occur in non-conformities, creating and implementing corrective actions and reviewing requirements set out by both the QMS and ISO 9001.
Customer Satisfaction
We’ve talked about customer satisfaction quite a bit already, but this is next up on the list for the requirements of ISO and should be part of your documentation and records.
Your rating should be monitored monthly, at the very least. Various analysis techniques can be applied to review the data. Feedback — Failure or Success should be followed-up with customer communication.
As mentioned before, customer satisfaction directly effects sales and profit — which should always be your goal for growing the business.
Improvement
Last, but not least, in the quality manual — the last of the mandatory requirements, we have improvement. The quality manager is one of the more important roles involved in this section, responsible for using evaluation tools to assess the QMS and develop recommendations for improvements.
Your organization should also make sure that the data that is analyzed is relevant to your QMS and relates to both short-term and long-term improvement.
Non-Conformity And Corrective Action
The ISO 9001 quality manual ends on the note of non-conformities and discussing corrective actions that can address them. Any non-conformities found are brought to the attention of the quality manager. The records from these findings are then logged alongside recommendations for corrective actions to resolve them.
There are many types of data inputs that could relate to the success of your QMS, including supplier performance, internal and external audit results, and evaluation of risks and opportunities. The quality manager often checks these data inputs as they become available.
If there are changes required to ensure that there is continual improvement in your organization’s processes, the quality manager may recommend a series of corrective actions that will need to be performed before your next audit or performance evaluation. The success that stems from these corrective actions will be assessed through the management review process.
Creating A Quality Manual And Document Management
Now that you have a better idea of what the quality manual looks like and what it generally includes, it is now up to you to customize it to fit your business.
How Do You Prepare a Quality Manual?
In order to make sure your quality manual fits the requirements from your quality management system or ISO 9001, your quality manual should:
- Demonstrate proper roles are assigned for processes
- Provide clear, concise information throughout
- Be detailed, but not too long
- Break outs each clause
How Should I Assign Document Numbers And Their Sequence?
We use the same numbering sequence as ISO 9001 in our quality manuals. You are free to replace these with a system that works best for your staff and for the business — as long as the system is logical, documented and communicated, it should be more than adequate.
We find it easier for external auditors during certification audits if it is the same as ISO standards. The lead auditor and other internal auditors often comment it is easier for documentation and records to have the same number and sequence as the requirements of ISO 9001.
Documented Procedures
When implementing ISO, you will need to document the procedures involved in your quality management system, including:
- Preventive actions
- Corrective actions
- Control of non-conforming products
- Control of documents
- Control of records
- Internal audit
You also need to document your main operating procedures or processes for your QMS.
Some companies recommend documenting lots of operating procedures — some Quality Manual Templates include more than 40 procedures! Our advice is to NOT make life so complicated for yourself, there is no need to document all these procedures, keep it simple and document fewer. Our ISO 9001 Quality Manual Template for example, comes with 10 procedures; this Template is suitable for all business types, large and small.
How Many Procedures Should You Document?
ISO 9000:2015 (3.4.5) defines a procedure as a specified way to carry out an activity or a process. With this in mind, businesses often risk becoming overburdened with too many procedures that document each business activity. This cumbersome methodology detracts from the 'process approach' that is a central theme in ISO 9001:2015.
We Recommend Documenting The Mandatory Processes
No matter how large or small your organization, by documenting the mandatory QMS processes, our documented procedures provide what you need to generate the records needed to ensure the effective operation and control of your organization's QMS and its processes. Our templates fulfill all the requirements of ISO 9001:2015.
Free download - Control of Calibrated Equipment procedure (ISO 9001)
The above free download will give you an idea of the current level of documentation required for an ISO 9001 procedure.
Quality Management Manual Template
Why Would You Document More Procedures?
Some ISO consultants and professionals recommend documenting many, many processes and procedures, we see some Templates with over 40 procedures!
We do not agree with this. It is not necessary to document all your business processes to achieve ISO 9001.
- How much more work do you want to give consultants and Certification Auditors? More procedures take more time and effort, and costs more to review. Why give auditors more information and more opportunities to find non-conformities?
- How many of your Clients want to wade through dozens of procedures to find the sections or elements that matter most to them? Keep it simple, for their benefit and yours!
- How much training can you do? As part of each procedure's implementation, employees require regular training, familiarization and monitoring. Lots of procedures will take more time away from the coal face!
Do not create too much documented information with too many procedures. You are going to be creating more work for yourself and everybody. It is going to take more time and it is going to cost more money.
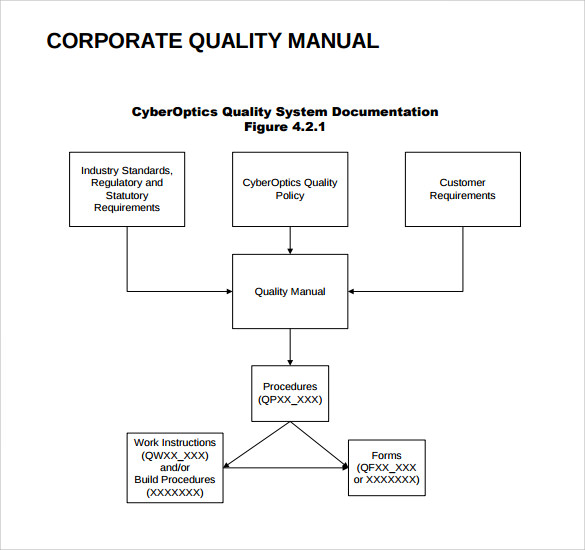
Process Maps — Description of Interaction of Processes
Represented by a flow chart, this description helps showcase each and every process within your company and how each one is connected to the other. This really is quite beneficial, as it does a great job of tying everything up and providing clarity if there is still any confusion left.
The description also makes the processes clear and concise to employees as well, which is important in ensuring they understand how to perform their tasks efficiently.
Example Process Map
The process map image is too big to insert here, Sorry!
Free downloads - 4 example process maps
PRO TIPS!
- Ask your business partners and suppliers for a PDF of their quality manual. You will find these invaluable and will they will help Top Management envisage the work to do.
- The business will find it beneficial if your quality manual is compatible with your most trusted business partners; try to develop your documentation to follow a similar outline, if possible.
Writing Style
Ensure your documents are easy to read and understand, do not use jargon specific to your industry. Write in a professional, informative and concise manner. Use short, broken up sentences, with informing large images.
Finally, your manual should be presented in a professional manner. Use your branding for the design and layout (this is where Templates that use styles, such as ours, are ideal). Distribute in PDF format, always with the date and/or version number in the filename or metadata.
Final Tips for Creating Your Manual
If you find that your organization is struggling to create a quality manual, consider these tips:
- To pass a certification body audit, a Quality Management System must be an audit-able entity
- Just writing about it is not enough — the QMS must also be implemented
- Aim for minimal documentation (especially if you're a small business). Don't document 40+ procedures — you're creating too much work for everyone
- Start with the assumption that you're currently doing all (or most) of what ISO 9001 asks
- Use separate documents for each procedure
- Use the same numbering as the standard
- PRO TIP! Ask your business partners, suppliers and customers for a PDF copy of their Quality Manual
- Use a Quality Manual Template — start your documentation out on the right track
- Create a list of policies that your organization needs. Think about what requirements you need to meet ISO 9001 standards in addition to what helps fulfill your quality management system’s needs
- Draft policies based on ISO requirements. These will be the most important, so make sure that you continuously review and revise them as needed until they are compliant
- Make sure that any terms specific to your company or industry are defined in a glossary or appendix within the manual; never assume that everyone will understand those business-specific terms
- Determine a general format for your quality manual and create a few first drafts to review and tweak as needed. Allow management from all departments to review the manual to ensure that the final draft is accurate to their areas. Doing this will also help you identify any inadequacies in various sections
- Once your team has finished reviewing and have agreed on any revisions, obtain a formal approval for the final draft before releasing
- Revision Control is important — particularly if many people are involved with contributing to different documents. Always document the revision date
- Make sure your official quality manual stays a controlled document as per ISO 9001 requirements. Update the document as your business needs change, and keep the language simple and easy for anyone who has access to it to understand
- Finally, ensure your quality manual is relevant to your organization; your quality manual is unique to your business and your business alone
Whether your business is looking to better satisfy ISO 9001 requirements or simply needs a way to ease the transition to a new quality management system, a quality manual can help. Although it seems complicated to create the documentation at first, it is quite straighforward to do with the right template in hand.
Further ISO 9001 Documentation
Updated: 5th September 2020
Author: Richard Keen
Richard Keen
Richard is our Compliance Director, responsible for content & product development.
But most importantly he is ISO's biggest fanboy and a true evangelist of the standards.
Learn more about Richard
More Information
FAQs About Our Templates
Can't Find What You're Looking For?
- Please use the Chat function at the bottom of the screen
- Enquiries [email protected]
- Support [email protected]
- Call 0845 054 2886 (UK)
Related searches
| ISO 9001 Procedures | ISO 14001 Procedures | ISO 45001 Procedures | Integrated Management System |
| ISO 9001 Internal Audit Checklist | ISO 14001 Internal Audit Checklist | ISO 45001 Internal Audit Checklist | ISO 9001 & 14001 |
| ISO 9001 Gap Analysis Checklist | ISO 14001 Gap Analysis Checklist | ISO 45001 Gap Analysis Checklist | ISO 9001 & 14001 & 45001 |
| Quality Manual Template | Environment Management System | Occupational Health & Safety | Internal Audits |
Overview
The quality manual template is a supplement to the laboratory quality management system training toolkit, Module 16 - Documents and records.
This quality manual template is based on internationally-accepted standards, and provides guidance for public health and clinical laboratories on writing policies and procedures that support a quality management system. It comprises a main document providing information and examples to assist with writing a laboratory quality manual, and 24 appendices (examples of standard operating procedures, forms, and processes). All documents are in Word format because they are meant to serve as templates and are thus modifiable. The individual laboratories are required to customize the text of the template to the local situation.
Quality Manual Samples
Download all the appendices
Iso 9001 Quality Manual Template
Iso 9001 Quality Management System Manual Template
Related links